 Aging Cell’s Best Paper Prize for 2017 honored the efforts of numerous McGowan Institute for Regenerative Medicine affiliated faculty members. The paper entitled “Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion,” appeared in the June 2017 issue of Aging Cell. The prize is awarded annually by the Anatomical Society on the recommendation of the Editor-in-Chiefs to the Lead Author and Co-Authors and is awarded to the paper considered to be the most outstanding publication in Aging Cell in that year by either a member or non-member of the Society.
Aging Cell’s Best Paper Prize for 2017 honored the efforts of numerous McGowan Institute for Regenerative Medicine affiliated faculty members. The paper entitled “Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion,” appeared in the June 2017 issue of Aging Cell. The prize is awarded annually by the Anatomical Society on the recommendation of the Editor-in-Chiefs to the Lead Author and Co-Authors and is awarded to the paper considered to be the most outstanding publication in Aging Cell in that year by either a member or non-member of the Society.
The McGowan Institute affiliated faculty members (in alphabetical order) who are co-authors of this work include:
- Fabrisia Ambrosio, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh with secondary appointments in the Departments of Physical Therapy, Bioengineering, Orthopaedic Surgery, and Microbiology & Molecular Genetics
- Antonio D’Amore, PhD, Research Assistant Professor in the Departments of Surgery and Bioengineering at the University of Pittsburgh
- Thomas Rando, MD, PhD, Professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, and the Director, Glenn Laboratories for the Biology of Aging, Stanford University, and the Deputy Director, Stanford Center on Longevity, also at Stanford University
- Rocky Tuan, PhD, Vice-Chancellor and President of The Chinese University of Hong Kong
- David Vorp, PhD, Associate Dean for Research, Swanson School of Engineering, University of Pittsburgh, and the John A. Swanson Professor of Bioengineering, with secondary appointments in the Departments of Cardiothoracic Surgery, Surgery, and the Clinical & Translational Sciences Institute at the University of Pittsburgh
- William Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine as well as a Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh
Student authors include Drs. Kristen M. Stearns-Reider (formerly in Dr. Ambrosio’s lab and now at University of California, Los Angeles) and Alkiviadis Tsamis (formerly of Carnegie Mellon University and Pitt).
The paper summary reads:
Age‐related declines in skeletal muscle regeneration have been attributed to muscle stem cell (MuSC) dysfunction. Aged MuSCs display a fibrogenic conversion, leading to fibrosis and impaired recovery after injury. Although studies have demonstrated the influence of in vitro substrate characteristics on stem cell fate, whether and how aging of the extracellular matrix (ECM) affects stem cell behavior has not been investigated. Here, we investigated the direct effect of the aged muscle ECM on MuSC lineage specification. Quantification of ECM topology and muscle mechanical properties reveals decreased collagen tortuosity and muscle stiffening with increasing age. Age‐related ECM alterations directly disrupt MuSC responses, and MuSCs seeded ex vivo onto decellularized ECM constructs derived from aged muscle display increased expression of fibrogenic markers and decreased myogenicity, compared to MuSCs seeded onto young ECM. This fibrogenic conversion is recapitulated in vitro when MuSCs are seeded directly onto matrices elaborated by aged fibroblasts. When compared to young fibroblasts, fibroblasts isolated from aged muscle display increased nuclear levels of the mechanosensors, Yes‐associated protein (YAP)/transcriptional coactivator with PDZ‐binding motif (TAZ), consistent with exposure to a stiff microenvironment in vivo. Accordingly, preconditioning of young fibroblasts by seeding them onto a substrate engineered to mimic the stiffness of aged muscle increases YAP/TAZ nuclear translocation and promotes secretion of a matrix that favors MuSC fibrogenesis. The findings here suggest that an age‐related increase in muscle stiffness drives YAP/TAZ‐mediated pathogenic expression of matricellular proteins by fibroblasts, ultimately disrupting MuSC fate.
Congratulations to all!
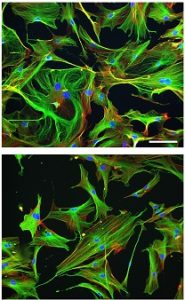
Illustration: Cytoskeletal phenotype of young and old skeletal muscle fibroblasts. Aging Cell.
Read more…
