August 2018 | VOL. 17, NO. 8| www.McGowan.pitt.edu
Platelet-Rich Plasma Does Not Promote Stem Cell-Mediated Cartilage Repair

Platelet-rich plasma (PRP) is believed to provide pain relief and help to improve joint function in degenerative joint disease, but a new study—coauthored by McGowan Institute for Regenerative Medicine affiliated faculty member Rocky Tuan, PhD, Vice-Chancellor and President of The Chinese University of Hong Kong—has shown that it does not act by promoting stem cell proliferation or enhance the cartilage formation capabilities of mesenchymal stem cells (MSCs). The effects of PRP treatment on cartilage formation and chondrogenesis in the presence of adult human MSCs derived from two different sources are reported in the study published in Tissue Engineering, Part A, a peer-reviewed journal from Mary Ann Liebert, Inc., publishers.
In the article entitled “Effect of Platelet-rich Plasma on Chondrogenesis of Adipose- and Bone Marrow-Derived Stem Cells,” coauthors Jr-Jium Liou, Benjamin Rothrauff, Peter Alexander, and Dr. Tuan, University of Pittsburgh School of Medicine, used MSCs derived from the fat pad of the knee and from the bone marrow. They showed that high concentrations of PRP treatment for long periods of time actually impaired cartilage formation, making it less likely for chondrocyte differentiation from the MSC to occur. This had important implications for the development of future strategies to repair cartilage damaged by injury or disease.
“This article presents a systematic study to elucidate the effects of PRP on the chondrogenic differentiation of adult human MSCs and its potential mechanism of action as a therapeutic adjunct for the treatment of joint diseases,” says Tissue Engineering Co-Editor-in-Chief Antonios Mikos, PhD, Louis Calder Professor at Rice University, Houston, Texas.
RESOURCES AT THE MCGOWAN INSTITUTE
September Histology Special
Make no Bones about it! The McGowan Histology Core has a Bone to pick FOR YOU!
The Histology lab offers decalcification, processing and embedding, cutting and staining of those difficult boney samples. Our staff has years of experience with bone samples that include special staining and IHC on sometimes difficult to work with, large and small bone samples. We have even developed a technique to insure your sample will not lift from the slide during aggressive antigen retrieval protocols.
Enjoy these beautiful examples, of just some of the special stains we offer at the McGowan Histology Core.
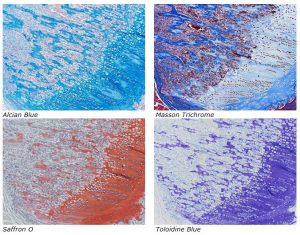
You’ll receive 25% off bone samples in September when you mention this ad. Contact Lori at the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or call 412-624-5265. As always, you will receive the highest quality histology in the lowest amount of time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
UPCOMING EVENTS
7th Annual Internal Symposium on Regenerative Rehabilitation Symposium

The Symposium on Regenerative Rehabilitation is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world, bringing together two disparate disciplines, regenerative medicine and rehabilitative research, into one synergistic field of science. Innovative ideas, concepts and technologies were shared, deliberated and debated at this premier event. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
The Symposium will be held October 11-13, 2018 in Seattle, Washington.
SCIENTIFIC ADVANCES
Insight to the Eye Lens

If you want to take clear photographs, you don’t use sandpaper to clear a smudge from your camera’s lens. Similarly, if you want to see clearly, the lens of your eye has to be free of obstruction.
For that reason, a curious thing happens during the development of eye lenses. Instead of closely guarding their nucleus and the DNA it contains – which normal cells do – most lens cells do the exact opposite. They actually degrade their own nuclei and other cell parts. If they were left in place, they would block clear vision causing cataracts at birth. Cataract disease, commonly found in the elderly, is a leading cause of blindness in the world.
Until now, scientists did not understand much about how these cells managed to do this – how they simultaneously pruned away obstructions without damaging proper development of the eye lens.
But now, in an article published in PLOS Genetics, University of Delaware biologist Salil Lachke, PhD, and his collaborators show the protein (Celf1) is essential to this process and reveal the mechanism used in three different vertebrates – mice, frogs and zebrafish. McGowan Institute for Regenerative Medicine affiliated faculty member Jeffrey Gross, PhD, E. Ronald Salvitti Chair in Ophthalmology Research in the Department of Ophthalmology, University of Pittsburgh School of Medicine, and the Vice-Chair and Director of Research, the Director of the Louis J. Fox Center for Vision Restoration and of the Ocular Development, Disease, and Regeneration Laboratory at the University of Pittsburgh School of Medicine, is a co-author on the report and he and his team studied zebrafish while the other collaborators studied mice and frogs.
The discoveries plant one of the earliest flags in a little-explored frontier of eye lens biology – showing how an RNA-binding protein found in fish, amphibians and mammals rewires components normally involved in cell division in order to control cell differentiation and specialization during lens development. They show that this RNA-binding protein is essential to breaking down the nucleus’ membrane and starting the process of digesting fiber cell DNA that would otherwise obstruct vision.
The study reveals how building the eye actually requires de-constructing the internal, opaque, structures inside the cells that form the lens, including destroying the DNA itself. But how does a cell degrade its own nucleus and DNA without killing itself and defeating the goal of lens development? How does it know when to stop this degradation?
The RNA-binding protein Celf1 is the key to this process.
“The functions of RNA-binding proteins, especially in organ development, are not well understood, mainly because scientists have not looked at them as carefully yet,” Dr. Lachke said.
“Most studies focus on signaling proteins and DNA-binding proteins that are important for initiating gene expression. But after you turn on a gene, you have to do a lot to precisely regulate its final output. In a process known as ‘post-transcriptional control,’ RNA-binding proteins determine the life span of their target RNA or whether that RNA should be translated into protein. Thus, it is essential to study RNA-binding proteins and the molecular processes they control after a gene is turned on.”
If you think of genes as lights that are turned on in a house, you could think of these proteins as the ones who go around making sure lights are kept on in the correct rooms and turned off when no longer needed.
That’s where Celf1 comes in. Its molecular interactions with specific RNA in the lens initiate events that make it possible to break open the envelope of the nucleus and degrade the DNA within in order to allow light to reach the retina properly.
When Celf1 is absent or deficient, serious eye disorders, including cataracts at birth, result.
“The whole process is very complicated and regulated very tightly,” said Archana Siddam, PhD, who recently earned her doctorate in Dr. Lachke’s lab and was the co-lead author on the PLOS Genetics article. “And this project sheds light on the molecular mechanism behind this complex process. We knew the lens gets rid of the nucleus, but we didn’t know the control mechanism. This sheds light on how exactly it is degraded, what genes are involved and that Celf1 is the factor that orchestrates the whole process.”
Part of the same process is critical to cell division and development – but in those cases it does not degrade the nucleus. Instead, it remodels its envelope so that the duplicated DNA can be evenly distributed to two daughter cells. This new information about Celf1 is sure to provide new traction in other cellular research, including cancer studies.
Neural Stem Cells Are the Key to Regeneration
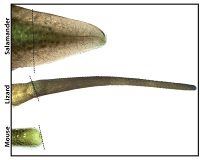
Cut off a salamander’s tail and, in a few weeks, a near-perfect replacement grows. Do the same to a lizard and a new tail will regrow, but it won’t be the same as the original. By comparing tail regeneration between the two animals, researchers at the University of Pittsburgh School of Medicine found that stem cells in the spinal cord are the ultimate limiting factor. McGowan Institute for Regenerative Medicine affiliated faculty members Thomas Lozito, PhD, Assistant Professor in Pitt’s Department of Orthopaedic Surgery and the Center for Cellular and Molecular Engineering, and Rocky Tuan, PhD, Vice-Chancellor and President of The Chinese University of Hong Kong, are co-authors on the study.
This finding, published in the Proceedings of the National Academy of Sciences, answers the longstanding question of why tail regeneration is perfect in the salamander and imperfect in the lizard, and may serve as a stepping stone to understanding why mice can’t regenerate their tails at all.
“The traditional animal model for regeneration is the salamander,” said senior author Dr. Lozito. “Salamanders can regenerate a wide variety of tissues – brain, heart, parts of their eyes, limbs, tails – but they have whole classes of molecule types and tissues that just aren’t found in mammals, so we really haven’t been able to apply very much of what we found in the salamander to humans.”
Understanding what separates perfect regeneration in the salamander from imperfect regeneration in the lizard lays the groundwork for bridging the gap to non-regenerating species, Dr. Lozito said.
They found the transplanted salamander stem cells retained their ability to differentiate into multiple cell types, including neurons. By contrast, lizard neural stem cells could become only glial cells, which don’t process the messages that direct movement and feeling.
The research team found that the traditionally described “neural stem cells” driving regeneration in the lizard are not “true” neural stem cells at all. Although they check many of the classic boxes, they fall short of a defining characteristic – the ability to spring forth a diversity of cell types.
That explains why there isn’t perfect tail regeneration in the lizard, Dr. Lozito said. The neural stem cells can’t produce the different cell types that would be needed to recreate the asymmetries of the original spinal cord, which in turn stymies the development of bony vertebrae.
“The spinal cord is the master regulator of tail regeneration, and these differences that we’re seeing between lizard and salamander tails are due to differences in stem cell quality,” Dr. Lozito said. “It’s all because of the neural stem cells.”
Additional authors on this study include Ricardo Londono, MD, PhD, and Megan Hudnall, BS, both of Pitt’s Center for Cellular and Molecular Engineering.
This research was funded by National Institutes of Health grant R01-GM115444.
Illustration: Salamanders tails regenerate perfectly, whereas lizard tails grow back imperfectly, and mouse tails don’t grow back at all. Thomas Lozito.
Integrated Sensor Could Monitor Brain Aneurysm Treatment

Implantation of a stent-like flow diverter can offer one option for less invasive treatment of brain aneurysms – bulges in blood vessels – but the procedure requires frequent monitoring while the vessels heal. Now, a multi-university research team—including McGowan Institute for Regenerative Medicine affiliated faculty members Youngjae Chun, PhD, associate professor, industrial engineering and bioengineering, and William Wagner, PhD, director of the McGowan Institute and professor of surgery, bioengineering and chemical engineering —has demonstrated proof-of-concept for a highly flexible and stretchable sensor that could be integrated with the flow diverter to monitor hemodynamics in a blood vessel without costly diagnostic procedures.
The sensor, which uses capacitance changes to measure blood flow, could reduce the need for testing to monitor the flow through the diverter. Researchers, led by Georgia Tech, have shown that the sensor accurately measures fluid flow in animal blood vessels in vitro, and are working on the next challenge: wireless operation that could allow in vivo testing.
The research was reported in the journal, ACS Nano, and was supported by multiple grants from Georgia Tech’s Institute for Electronics and Nanotechnology, the University of Pittsburgh and the Korea Institute of Materials Science.
“The nanostructured sensor system could provide advantages for patients, including a less invasive aneurysm treatment and an active monitoring capability,” said Woon-Hong Yeo, an assistant professor in Georgia Tech’s George W. Woodruff School of Mechanical Engineering and Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “The integrated system could provide active monitoring of hemodynamics after surgery, allowing the doctor to follow up with quantitative measurement of how well the flow diverter is working in the treatment.”
“We have developed a highly stretchable, hyper-elastic flow diverter using a highly-porous thin film nitinol,” Dr. Chun explained. “None of the existing flow diverters, however, provide quantitative, real-time monitoring of hemodynamics within the sac of cerebral aneurysm. Through the collaboration with Dr. Yeo’s group at Georgia Tech, we have developed a smart flow-diverter system that can actively monitor the flow alterations during and after surgery.”
Repairing the damaged artery takes months or even years, during which the flow diverter must be monitored using MRI and angiogram technology, which is costly and involves injection of a magnetic dye into the blood stream. Dr. Yeo and his colleagues hope their sensor could provide simpler monitoring in a doctor’s office using a wireless inductive coil to send electromagnetic energy through the sensor. By measuring how the energy’s resonant frequency changes as it passes through the sensor, the system could measure blood flow changes into the sac.
Because the brain’s blood vessels are so small, the flow diverters can be no more than five to ten millimeters long and a few millimeters in diameter. That rules out the use of conventional sensors with rigid and bulky electronic circuits.
“Putting functional materials and circuits into something that size is pretty much impossible right now,” Dr. Yeo said. “What we are doing is very challenging based on conventional materials and design strategies.”
The researchers tested three materials for their sensors: gold, magnesium and the nickel-titanium alloy known as nitinol. All can be safely used in the body, but magnesium offers the potential to be dissolved into the bloodstream after it is no longer needed.
The proof-of-principle sensor was connected to a guide wire in the in vitro testing, but Dr. Yeo and his colleagues are now working on a wireless version that could be implanted in a living animal model. While implantable sensors are being used clinically to monitor abdominal blood vessels, application in the brain creates significant challenges.
“The sensor has to be completely compressed for placement, so it must be capable of stretching 300 or 400 percent,” said Dr. Yeo. “The sensor structure has to be able to endure that kind of handling while being conformable and bending to fit inside the blood vessel.”
Illustration: With gloved fingers for scale, a proof-of-concept flow sensor is shown here on a stent backbone. Credit: Woon-Hong Yeo, Georgia Tech.
New Leadership Roles for Dr. George Gittes

McGowan Institute for Regenerative Medicine affiliated faculty member George Gittes, MD, a nationally recognized pediatric surgeon and well-established basic scientist, has been appointed director of the Richard King Mellon Foundation Institute for Pediatric Research and co-scientific director at UPMC Children’s Hospital. He also will assume the title of surgeon-in-chief emeritus.
Since joining UPMC Children’s in 2005, Dr. Gittes has served as surgeon-in-chief and chief of the Division of Pediatric General and Thoracic Surgery — one of the busiest pediatric surgical programs in the United States and the home of the Benedum Pediatric Trauma Program, one of only two Level I Regional Resource Pediatric Trauma Centers in Pennsylvania.
Dr. Gittes, professor of surgery and pediatrics, Pitt School of Medicine and the Benjamin R. Fisher Chair in Pediatric Surgery, will lead the Mellon Institute, which is an incubator for research that challenges conventional wisdom and can lead to paradigm shifts in pediatric medicine. The high-risk, high-impact investigations undertaken at the institute are typically not funded through government or conventional sources, placing Children’s in a unique realm of pediatric research centers.
And as co-scientific director, Dr. Gittes will work alongside Terence Dermody, MD, chair of the Department of Pediatrics at the Pitt School of Medicine and physician-in-chief and scientific director at UPMC Children’s, to further enhance the hospital’s already robust and historically significant pediatric research program.
In his own lab, Dr. Gittes and his team have been performing studies using gene therapy to treat diabetes. His team has found that a virus injected into the pancreatic duct of mice will reverse their diabetes, leading to the potential to change the treatment of juvenile diabetes.
Needed: A Moonshot for Sepsis

In his opinion piece for STAT, McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Chair of the Department of Critical Care Medicine of both the University of Pittsburgh School of Medicine and the UPMC Healthcare System, states:
“Sepsis is the most expensive and deadliest condition we are battling in our hospitals. But when did you last see headlines trumpeting a blockbuster new sepsis drug? You haven’t — and you won’t — until we make a serious commitment to fight the oldest disease of all.”
Sepsis is an infection that kills as many Americans each year — about 250,000 — as stroke and Alzheimer’s combined. Nearly 1.5 million Americans are hospitalized for sepsis each year, and it accounts for 1 in 3 deaths that occur in hospitals.
Sepsis is the syndrome of an acute, life-threatening failure of the body’s vital organs that develops as the body tries to fight off infection. It can start with something simple: an infected cut, the flu, a urinary tract infection, or many other seemingly benign afflictions. It is a case of friendly fire, where the body’s own immune system and other defense mechanisms respond clumsily to the infection, accidentally injuring or interfering with vital organs.
The onset of sepsis is pernicious. An individual can look relatively well until it is too late to intervene and stop the organ-destroying cascade of an overreactive immune system.
Dr. Angus’ proposed plan to solve sepsis includes meeting these needs:
- better methods to identify sepsis early and accurately
- better treatments and strategies to care for patients, not just newer antibiotics
- better ways to help and treat sepsis survivors
Many of the tools that might be useful to truly reduce sepsis exist but are scattered across hospital and research institutions. Today, there is no coordinated effort to bring together researchers, industry, hospitals, and patients. Sepsis is an old term. It needs a new examination.
At the University, Dr. Angus holds the rank of Distinguished Professor and the Mitchell P. Fink Endowed Chair in Critical Care Medicine with secondary appointments in Medicine, Health Policy and Management, and Clinical and Translational Science and he directs the CRISMA (Clinical Research, Investigation, and Systems Modeling of Acute Illnesses) Center. He also co-directs the UPMC ICU Service Center, responsible for the provision of ICU services across the 20-plus hospital system.
Dr. Joseph Glorioso Receives ACGT Grant in Support of a Cancer Vaccine for Melanoma

Alliance for Cancer Gene Therapy (ACGT), the nation’s only charitable organization dedicated exclusively to funding cancer cell and gene therapy research, announced the recipients of its 2018 research grants using innovative clinical approaches to find gene therapy treatments for solid cancers. One of the projects funded is a collaborative effort by McGowan Institute for Regenerative Medicine affiliated faculty member Joseph Glorioso, MD, PhD, Professor in the Department of Microbiology and Molecular Genetics of the University of Pittsburgh, and Gary Cohen, PhD, of the University of Pennsylvania, who are working together on a vaccine for melanoma.
Drs. Glorioso and Cohen will share a $500,000, two-year ACGT grant to support a study to develop a cancer vaccine for melanoma. Their research builds on previously successful results using a tumor-targeted, actively replicating herpes virus to infiltrate cancers and stimulate an immune system assault. Dr. Glorioso calls the methodology a “heat-seeking missile that targets metastatic cancer for destruction.” The treatment doesn’t stop there. Once the cancer is eliminated, the vaccine inserts an immunity barrier to protect against recurrence. Melanoma is among the most deadly cancers and this treatment offers new hope.
Established in 2001, ACGT is the nation’s only non-profit dedicated exclusively to funding cell and gene therapy research for all types of cancer. One hundred percent of contributions go directly to research. Since its inception, ACGT has funded some of the underlying science that has resulted in the formation of either licensing agreements or biotech companies including Novartis, Ziopharm, Juno, Tmunity, Turnstone Biologics, all of which are in various stages of bringing new treatments to patients. ACGT has funded 58 grants in the U.S. and Canada to conduct and accelerate critically needed innovative research. 36 of those grants have gone to Young Investigators for basic research and 22 grants to Clinical Investigators, totaling more than $29 million in funding.
Pitt’s Center for Medical Innovation Awards Funding to Four Projects of McGowan Institute Affiliated Faculty Members

The University of Pittsburgh’s Center for Medical Innovation (CMI) awarded grants totaling $105,000 to five research groups through its 2018 Round-1 Pilot Funding Program for Early Stage Medical Technology Research and Development. Four of these research groups involve the expertise of McGowan Institute for Regenerative Medicine affiliated faculty members; the projects include:
- a new vascular access device for use with stent grafts,
- an artificial tricuspid valve for treatment of right-heart disease,
- a biological treatment for inflammatory bowel disease, and
- a biofeedback system for mobility rehabilitation training.
This is our seventh year of pilot funding, and our leadership team could not be more excited with the breadth and depth of this round’s awardees,” said McGowan Institute affiliated faculty member Alan Hirschman, PhD, CMI Executive Director. “This early-stage interdisciplinary research helps to develop highly specific biomedical technologies through a proven strategy of linking UPMC’s clinicians and surgeons with the Swanson School’s engineering faculty.
“E-mag system for rapid cannulation of fenestrated stent grafts to reduce radiation exposure”
For the development of a vascular stent graft system that will magnetically guide cannulation of endograft branches.
- Bryan Tillman, MD, PhD, Division of Vascular Surgery Department of Surgery, University of Pittsburgh Medical Center
- Youngjae Chun, PhD, Associate Professor, Industrial Engineering, Swanson School of Engineering
“Valved stent conduit for the treatment of severe advanced tricuspid regurgitation”
For the development of an artificial tricuspid valve that will treat decreased right ventricular performance due to cardiac disease.
- Catalin Toma, MD, Assistant Professor, University of Pittsburgh School of Medicine Heart and Vascular Institute
- Youngjae Chun, PhD, Associate Professor, Industrial Engineering, Swanson School of Engineering
“Local induction of tolerogenic T cells to ameliorate inflammation in inflammatory bowel disease”
For the development of a potent IBD therapy with fewer side effects than current medical therapy.
- Warren Sands, MD, PhD, T32 Clinical and Research Fellow, Division of Gastroenterology, Hepatology, and Nutrition at the University of Pittsburgh Medical School
- Steven Little, PhD, William Kepler Whiteford Endowed Professor and Chair, Department of Chemical and Petroleum Engineering, Swanson School of Engineering
- David Binion, MD, AGAF, FACG, Professor of Medicine, Clinical and Translational Science Division of Gastroenterology, Hepatology, and Nutrition, University of Pittsburgh Medical School
“MOVISU-FIT: Mobile Wearable System for Real Time Visual Feedback and Gait Training”
For the development of a system to provide real-time visual feedback to patients working on gait corrections during mobility rehabilitation training.
- Goeran Fiedler, PhD, Assistant Professor, Rehabilitation Science and Technology, UPMC
- William Clark, PhD, Professor, Mechanical Engineering and Materials Science, Swanson School of Engineering
- David Brienza, PhD, Professor, School of Health and Rehabilitation Sciences
- Krista Kutina, DPT, Researcher, School of Health and Rehabilitation Sciences
- Alicia Koontz, PhD, Associate Professor, Veterans Administration Hospital
- April Chambers, PhD, Research Assistant Professor, Bioengineering, Swanson School of Engineering
CMI, a University Center housed in Pitt’s Swanson School of Engineering (SSOE), supports applied technology projects in the early stages of development with “kickstart” funding toward the goal of transitioning the research to clinical adoption. Proposals are evaluated on the basis of scientific merit, technical and clinical relevance, potential health care impact and significance, experience of the investigators, and potential in obtaining further financial investment to translate the particular solution to healthcare.
Congratulations, all!
Illustration: University of Pittsburgh Swanson School of Engineering.
Dr. Kang Kim to Collaborate on National Kidney Foundation Award

National Kidney Foundation (NKF) has selected its award recipients of the 2018 NKF Young Investigator Research Grant Program, which strives to improve the quality of life for people with kidney disease by funding promising young scientists in their research to discover the causes of kidney disease, prevent its progression, and improve treatment for those living with it today. One of the projects selected includes the expertise of McGowan Institute for Regenerative Medicine affiliated faculty member Kang Kim, PhD, Associate Professor of Medicine and Bioengineering at the University of Pittsburgh and the Heart and Vascular Institute at UPMC.
Nephrologist Roderick Tan, MD, PhD, was awarded the Edith H. Blattner Grant Young Investigator Grant for research that will utilize high-resolution ultrasound to closely examine the kidney’s vital small blood vessels. Dr. Tan is Assistant Professor of Medicine, Division of Renal-Electrolyte, Department of Medicine at the University of Pittsburgh. He is collaborating on the study with colleague Dr. Kim.
The grants are awarded in specified categories for one-year terms. They are given based upon careful and balanced peer review by an independent committee with an emphasis on the support of high-quality, clinical investigation.
The NKF Young Investigator Research Program exists to help innovative researchers reach potentially pioneering results. “We strive to stay ahead of the curve when it comes to finding new and better ways to fight or treat kidney disease,” said Joseph Vassalotti, MD, and Chief Medical Officer of the National Kidney Foundation. “Ultimately, it’s about improving the lives of millions of kidney patients, so it is with great responsibility and pride that we support the vital research of these doctors.”
AWARDS AND RECOGNITION
Pitt ChemE Team Receives HHMI Gilliam Fellowship

A new group of graduate students and their advisers is joining the drive to increase diversity and inclusion in science. Emily Ackerman, PhD student, and her adviser, McGowan Institute for Regenerative Medicine affiliated faculty member Jason Shoemaker, PhD, Assistant Professor, Department of Chemical & Petroleum Engineering, University of Pittsburgh, is one of those student-adviser pairs. Ms. Ackerman is a PhD student studying network virology and host immune responses in Dr. Shoemaker’s lab.
The Howard Hughes Medical Institute (HHMI) has awarded 2018 Gilliam Fellowships for Advanced Study to 45 doctoral student-adviser pairs from across the country. All have demonstrated high promise to become leaders in their fields, says David Asai, PhD, HHMI’s senior director for science education. “These are incredibly talented young scientists with the desire to become college and university faculty, where they will help shape the next generation students,” Dr. Asai says.
HHMI created the Gilliam Fellowships for Advanced Study in honor of the late James H. Gilliam, Jr. A charter trustee of HHMI, Mr. Gilliam was a respected business and civic leader who spent his life nurturing excellence and diversity in science and education.
This year, HHMI received 231 applications for Gilliam Fellowships. Selection criteria include nominating materials from the student’s university, the student’s research proposal, a leadership statement, a letter of recommendation, and the adviser’s mentoring plan and proposal for a diversity and inclusion activity.
To be eligible, students must be enrolled in their second or third year of a PhD program in biomedical or life sciences disciplines, but not in an MD/PhD program. Students must be eligible for grants awarded through the National Institute of General Medical Sciences (NIGMS) and must be from racial, ethnic, or other underrepresented groups in the sciences or be alumni of the HHMI EXROP program. Students also must aspire to careers in academic science and demonstrate a commitment to the advancement of diversity and inclusion in the sciences.
The Gilliam program aims to ensure that a diverse and highly trained workforce is prepared to assume leadership roles in science, he explains. HHMI is taking a two-pronged approach: supporting promising graduate students from groups that are underrepresented in science and helping their thesis advisers build inclusive training environments.
Each pair will receive an annual award totaling $50,000 – which includes a stipend, a training allowance, and an institutional allowance – for up to three years. Fellows’ thesis advisers will participate in a year of mentor development activities, including online training and two in-person workshops at HHMI headquarters in Chevy Chase, Maryland.
Congratulations, Ms. Ackerman and Dr. Shoemaker!
Vande Geest Lab Graduate Student Named BioE’s 2018 Leonard H. Berenfield Fellow

The University of Pittsburgh Department of Bioengineering selected Ali Behrangzade for its Leonard H. Berenfield Graduate Fellowship in Cardiovascular Bioengineering. This competitive fellowship is awarded to one student each academic year. Mr. Behrangzade is a graduate student in the Soft Tissue Biomechanics Lab, which is headed by McGowan Institute for Regenerative Medicine affiliated faculty member Jonathan Vande Geest, PhD, Professor, Department of Bioengineering.
Recipients of this award receive one year of funding for cardiovascular research performed in Pitt’s Swanson School of Engineering. They retain the title of Berenfield Fellow throughout their PhD studies and occasionally meet with the award’s donor.
Mr. Behrangzade earned his BSc and MSc degrees in mechanical engineering from the University of Tehran. During his master’s, he focused on experimental and computational fluid mechanics. He is currently pursuing a PhD in bioengineering under advisor Dr. Vande Geest.
Mr. Behrangzade is working on functional tissue-engineered vascular grafts (TEVGs) that will be used for coronary artery bypass graft (CABG) surgery. According to a 2015 American Heart Association report, coronary artery disease (CAD) occurs in 32.2 percent of males and 18.8 percent of females over the age of 80, and most of these patients require CABG surgery.
Autologous vessels are blood vessels obtained from the same individual and used in CABG surgery. However, these vessels are not always suitable because of prior harvesting or pre-existing vascular disease.
“Since current alternatives for autologous vessels lead to failure of the grafts via intimal hyperplasia – thickening of the inner layer of a blood vessel – and graft thrombosis, a functional TEVG is required for CABG surgery,” said Mr. Behrangzade. “As part of this project, I’m studying vasoactivity – the contraction and dilation of a blood vessel in response to different stimuli – of these vascular grafts which is a necessary feature of a functional TEVG.”
“Vasoactivity contributes to the regulation of blood pressure by a contraction/dilation process. Since smooth muscle cells (SMCs) play the key role in vasoconstriction/vasodilation, I am investigating the responsiveness of the SMC-seeded vascular graft to different chemical stimuli,” explained Mr. Behrangzade. “The ultimate goal of this research is to provide a functional TEVG as a reliable alternative for autologous vessels for CABG surgery, which will lead to less failure and can benefit patients and the healthcare system.
About Leonard H. Berenfield:
Leonard H. Berenfield received his bachelor’s degree in mechanical engineering from the University of Pittsburgh in 1964.
In 1965, after one year at Westinghouse, he moved to Warren, Pennsylvania, to use his engineering knowledge to help grow Berenfield Steel Drum Co. – the family steel drum manufacturing business. The firm’s continued growth led to reorganization as Berenfield Containers, Inc. in 1985 with Mr. Berenfield assuming the role of President. Further expansions of existing plants over the years and the acquisition of plants in Harrisburg, North Carolina, and Pine Bluff, Arkansas, as well as new factories to diversify the product line into fibre drums established the company’s legacy. Mauser USA purchased Berenfield Containers in 2016.
Mr. Berenfield was born and raised in the Pittsburgh area and is an active volunteer. He has held posts in several nonprofit and industry boards including the American Heart Association, the United Way, the Jewish Federation of Cincinnati, Hebrew Union College, the Steel Shipping Container Institute, the International Fibre Drum Institute, and the Industrial Steel Drum Institute.
In 2018, he was named Distinguished Alumnus of the Swanson School’s Department of Mechanical Engineering and Materials Science.
Congratulations, Mr. Behrangzade!
Illustration: University of Pittsburgh Swanson School of Engineering.
Dr. Ambrosio and Dr. Phillippi Join the McGowan Executive Committee

Fabrisia Ambrosio, PhD, MPT—Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh with secondary appointments in the Departments of Physical Therapy, Bioengineering, Orthopaedic Surgery, and Microbiology & Molecular Genetics—and Julie Phillippi, PhD—Assistant Professor in the Department of Cardiothoracic Surgery, School of Medicine, with a secondary appointment in the Department of Bioengineering, Swanson School of Engineering—have joined the McGowan Institute for Regenerative Medicine Executive Committee.
The Committee provides guidance to the Institute on strategic issues and opportunities. The Committee also reviews and approves all secondary faculty appointments in the Institute.
The other members of the Executive Committee are:
| Stephen Badylak, DVM, PhD, MD | Joerg Gerlach, MD, PhD | Donna Stolz, PhD |
| Harvey Borovetz, PhD | Robert Kormos, MD | Elizabeth Tyler-Kabara, MD, PhD |
| Bryan Brown, PhD | Eric Lagasse, PharmD, PhD | Yoram Vodovotz, PhD |
| Patrick Cantini | Kacey Marra, PhD | William Wagner, PhD |
| Cynthia Cavallucci | John Murphy | Alan Wells, MD, DMS |
| Ira Fox, MD | Darlene Noah | Stephen Winowich |
| Charles Sfeir, DDS, PhD |
2018 Wiegand Intern Completes Assignment

The Wiegand Summer Internship provides an opportunity for a high school senior, who resides in Allegheny County and who graduated in the spring of 2018, to spend 5 weeks learning first-hand about regenerative medicine research and scientific investigations in general.
The 2018 Wiegand Intern was Kaitlyn Wintruba a graduate of West Mifflin High School. Ms. Wintruba will be attending the Swanson School of Engineering, University of Pittsburgh in the Fall. Her internship was in the Thoracic Aortic Disease Research Laboratory under the mentorship of McGowan Institute for Regenerative Medicine faculty member Julie Philippi, PhD, Assistant Professor in the Department of Cardiothoracic Surgery, School of Medicine, University of Pittsburgh. Dr. Phillippi also has a secondary appointment in the Department of Bioengineering, Swanson School of Engineering.
Ms. Wintruba worked on a project focusing on The Influence of ECM Biomaterials on Human Smooth Muscle Cell Function. The purpose of the project was to develop a cell culture model to understand how human aortic smooth muscle cells respond to cues from the extracellular matrix.
The Wiegand Summer Internship is made possible through an endowment from Mr. and Mrs. Bruce Wiegand, friends and supporters of the McGowan Institute for Regenerative Medicine. The endowment is designed to provide an opportunity for a high school senior to experience the opportunities and excitement of a career in science and engineering, with a focus on regenerative medicine.
Illustration: Pictured are (L to R): Dr. Julie Philippi, Kaitlyn Wintruba
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#187 –– Professor Paul Fairchild discusses his research in developmental and stem cell biology. He also discuses his work with the Journal of Immunology and Regenerative Medicine.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Liou JJ, Rothrauff BB, Alexander PG, Tuan RS
Title: Effect of Platelet-rich Plasma on Chondrogenesis of Adipose- and Bone Marrow-Derived Stem Cells
Summary: Post-traumatic and focal cartilage defects of the knee affect over 3 million Americans annually. Autologous cell-based cartilage repair, for example, autologous chondrocyte implantation, is limited by the need for ex vivo chondrocyte expansion and donor site morbidity. Mesenchymal stem cells (MSCs), owing to their relative ease of isolation, higher replication activity, and chondrogenic potential, represent an alternative reparative cell type. Platelet-rich plasma (PRP) is an autologous, growth factor-rich biologic preparation that has recently received increasing attention and use as a therapeutic adjunct for the treatment of degenerative joint diseases, and there is evidence suggesting that PRP acts by promoting stem cell proliferation and tissue healing. In this study, we have examined the effect of PRP treatment on chondrogenic differentiation of adult human MSCs derived from infrapatellar fat pad-adipose stem cells (IFP-ASCs) and bone marrow (BM-MSCs). Both cell types were placed in high-density pellet culture and hydrogel-encapsulated culture under chondrogenic conditions. Our results showed that PRP did not improve IFP-ASC or BM-MSC chondrogenesis. In general, chondrogenesis was inhibited with increasing PRP concentrations and duration of exposure, on the basis of histological, biochemical, and gene expression analyses. Taken together, these findings suggest that although PRP is reported to be beneficial in terms of pain relief and joint function improvement, its mechanism of action is unlikely to directly involve enhancement of MSC-mediated hyaline cartilage formation.
Source: Tissue Eng Part A. 2018 Jul 23. [Epub ahead of print]
GRANT OF THE MONTH
PI: Joseph Glorioso and Gary Cohen, PhD
Title: Cancer Vaccine for Melanoma
Description: Drs. Glorioso and Cohen will share a two-year ACGT grant to support a study to develop a cancer vaccine for melanoma. Their research builds on previously successful results using a tumor-targeted, actively replicating herpes virus to infiltrate cancers and stimulate an immune system assault. Dr. Glorioso calls the methodology a “heat-seeking missile that targets metastatic cancer for destruction.” The treatment doesn’t stop there. Once the cancer is eliminated, the vaccine inserts an immunity barrier to protect against recurrence. Melanoma is among the most deadly cancers and this treatment offers new hope.
Source: Alliance for Cancer Gene Therapy
Amount: $500,000
Term: 3 Years
