April 2016 | VOL. 15, NO. 4| www.McGowan.pitt.edu
MIRM’s Technology for Organ and Tissue Preservation is TechConnect 2016 Award Winner
 The pioneering research led by Paulo Fontes, MD, FACS, UPMC transplant surgeon, associate professor, Starzl Transplantation Institute, Department of Surgery, Pitt School of Medicine, and a deputy director of the McGowan Institute in the development of a new hemoglobin-based oxygen carrier (HBOC) for organ and tissue preservation in combination with machine perfusion (MP) has been selected as a 2016 TechConnect Innovation Awardee. The TechConnect Innovation Awards selects the top early-stage innovations from around the world through an industry-review process of the top 15% of annually submitted technologies into the TechConnect National Innovation Summit. Rankings are based on the potential positive impact the submitted technology will have on a specific industry sector
The pioneering research led by Paulo Fontes, MD, FACS, UPMC transplant surgeon, associate professor, Starzl Transplantation Institute, Department of Surgery, Pitt School of Medicine, and a deputy director of the McGowan Institute in the development of a new hemoglobin-based oxygen carrier (HBOC) for organ and tissue preservation in combination with machine perfusion (MP) has been selected as a 2016 TechConnect Innovation Awardee. The TechConnect Innovation Awards selects the top early-stage innovations from around the world through an industry-review process of the top 15% of annually submitted technologies into the TechConnect National Innovation Summit. Rankings are based on the potential positive impact the submitted technology will have on a specific industry sector
A new HBOC molecule is being developed by VirTech Bio (VTB) LLC, Beverly, MA under the leadership of W. Richard Light, PhD in collaboration with faculty members from OSU, Columbus, OH. Dr. Light, a leading HBOC scientist, has been collaborating with Dr. Fontes for several years in the development of this new approach to promote effective ex-vivo oxygenation over an extended period of time. They initially developed and patented this technology in 2012, after a series of proof-of-concept pre-clinical experiments in liver transplantation at the McGowan Institute. They have further expanded this application for a discarded human liver and subsequently for vascularized composite allografts (VCA). The MP/HBOC technology is intended to enhance organ and tissue preservation prior to transplantation and it is expected to have a positive impact in clinical transplantation in the near future. Organ preservation is currently based on cold storage (CS), where the organs remain hypothermic and anoxic for a limited period of time. CS has a direct negative impact on organ quality and on the time and the distances imposed by the current system of organ allocation
The McGowan Institute team has signed a Corporate Research Agreement with VTB, which is intended to advance this technology through the current regulatory pathway for medical devices once an investigational device exemption (IDE) has been granted by the FDA. The group has published their original manuscript in the American Journal of Transplantation in 2015. Seven additional studies have been recently accepted to be presented at the American Transplant Congress, June 2016, Boston, MA, which includes some intriguing new findings about the ability of the MP/HBOC system to induce a hibernation-like state in swine liver allografts preserved at 21°C over a 9 hour period.
The MP/HBOC technology has been expanded into pre-clinical experiments with VCA and successfully presented at the European Society of Organ Transplantation in 2015. The VCA studies were initially sponsored by the Department of Defense (DOD) as a way to successfully complete the initial proof-of-concept experiments. Further developments with a full limb transplant model are currently underway at the Institute of Surgical Research, San Antonio Medical Center, San Antonio, TX, under the leadership of Lt Col Michael Davis, MD, FACS and in collaboration with Vijay Gorantla, MD. The same beneficial impact of effective ex-vivo oxygenation in subsequent tissue regeneration seen extensively in liver allografts has been further reproduced in VCA. These studies are aimed to provide better therapeutic options for limb rescue, replantation and transplantation in the near future.
Further collaborations are also underway with Yoram Vodovotz, PhD and his team, who have shown a beneficial effect of ex-vivo oxygenation in decreasing inflammatory networks when compared to CS. Dr. Vodovotz has introduced a novel analytic approach for the MP/HBOC ex-vivo system by performing principal component analysis and several forms of dynamic network analysis with inflammatory markers sampled during liver preservation. This computational biology approach has helped identify potentially novel anti-inflammatory effects of this novel organ preservation system.
Machine perfusion is already a clinical reality in kidney and lung transplantation, but still costly with some of the new devices used under normothermic (37°C) conditions and with red blood cells as their primary oxygen carrier component.
The TechConnect awards will be presented during the TechConnect-National Innovation Summit, Washington, D.C.; May 22-25, 2016.
RESOURCES AT THE MCGOWAN INSTITUTE
May Special at the Histology Lab
The Histology Stain of the Month: Toluidine blue
Mast cells play a key role in the inflammatory process. A mast cell is a myeloid derived cell and is part of the immune system. Mast cells contain many distinctive granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in angiogenesis, and defense against pathogens, and wound healing.
Toluidine blue is one of the most common stains for acid mucopolysaccharides and glycoaminoglycans, which are both components of mast cells granules.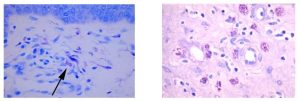
You’ll receive 20% off Toluidine blue Staining for the entire month of May when you mention this ad. Contact Lori at the McGowan Core Histology Lab by email: perezl@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology with the fastest turnaround time.
Shout out to the Flow Cytometry Core at the McGowan Institute
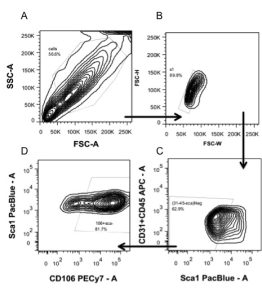
Dr. Fabrisia Ambrosio is an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh. She holds secondary appointments in the Departments of Physical Therapy, Orthopedic Surgery, and Microbiology &Molecular Genetics. In addition, she is a faculty member of the neurology residency program in the Department of Physical Therapy. Her long-term research goal is the development of Regenerative Rehabilitation approaches to improve the skeletal muscle healing and functional recovery. Her laboratory uses murine and human models to investigate the underlying mechanisms by which targeted and specific mechano-transductive signals can be used to enhance donor and/or host stem cell functionality.
My laboratory, which has primary research interests in muscle stem cells and skeletal muscle regeneration, has been working with Lynda for several years now. Using flow cytometry, we have successfully purified muscle satellite cells from mice at various ages and treatment conditions. These purified cells have been used to perform in vitro surrogate measures of skeletal muscle regenerative potential. Data collected from these analyses are currently being compiled for potential publication.
Over time, we have come to rely heavily on Lynda’s expertise to both isolate and characterize muscle stem cells (see sample gating strategy for muscle stem cell sorting below). Lynda has helped us at all stages of our experiments, from design, to troubleshooting, to interpretation of results, and even to assembling figures. Perhaps the greatest benefit that me and my laboratory have received is that Lynda is extremely meticulous and thorough, and she takes the time to discuss experiments in depth with the trainees in the laboratory, many of whom never had previous experience with flow cytometry. In cases when experiments fail, Lynda always meets with the trainees to troubleshoot steps for moving forward. Whenever possible, I also attend those meetings, as I learn so much.
Without a doubt, the Flow Cytometry Core is a tremendous asset to the McGowan Institute.
Contact Lynda Guzik, the Flow Cytometry Facility Manager, for more information on how the Facility’s resources can help you: 412-648-8660 or guzilj@upmc.edu.
UPCOMING EVENTS
Save the Date: Regenerative Rehabilitation Advanced Training Course
 The University of Pittsburgh and Magee-Womens Research Institute is hosting the NIH-sponsored Rehabilitative and Regenerative Medicine for Minority Health and Health Disparities Advanced Training Course from June 6-10, 2016. The purpose of this NICHD Continuing Education Training Program is to provide comprehensive and sophisticated training and state-of-the-art methods on bioengineering, cellular, molecular and genetic approaches for advancing the fields of Rehabilitative and Regenerative Medicine. Program organizers include Drs. Gerald Schatten, Fabrisia Ambrosio, and Michael Boninger. Researchers and clinicians from around the country have been selected to present during this week-long course, including Pioneer Award Lecturer, Dr. Donald Ingber from the Wyss Institute for Biologically Inspired Engineering at Harvard University.
The University of Pittsburgh and Magee-Womens Research Institute is hosting the NIH-sponsored Rehabilitative and Regenerative Medicine for Minority Health and Health Disparities Advanced Training Course from June 6-10, 2016. The purpose of this NICHD Continuing Education Training Program is to provide comprehensive and sophisticated training and state-of-the-art methods on bioengineering, cellular, molecular and genetic approaches for advancing the fields of Rehabilitative and Regenerative Medicine. Program organizers include Drs. Gerald Schatten, Fabrisia Ambrosio, and Michael Boninger. Researchers and clinicians from around the country have been selected to present during this week-long course, including Pioneer Award Lecturer, Dr. Donald Ingber from the Wyss Institute for Biologically Inspired Engineering at Harvard University.
This dynamic training course provides a series of daily lecturers on emerging concepts, followed by extended discussion, laboratory research, technologically intense workshops and informal seminars over a week-long period. The primary aim is to educate and update rehabilitation and regeneration researchers on the implications of stem cells and tissue engineering for mechanistic discoveries and on designing improved strategies for rehabilitation discoveries, especially in the CNS and skeletal-muscular systems. This course provides an innovative educational opportunity to motivate biomedical trainees to pursue scientific careers in rehabilitation and regenerative research research.
The target audience is young and promising faculty, as well as advanced postdoctoral fellows eager to learn about the newest findings and controversies. We intend to give participants knowledge of laboratory techniques, career mentoring and the ethical-legal-societal impact of rehabilitative and regenerative research to greatly enhance their successful entry into this field or to strengthen their knowledge in this field.
For more information, contact pdc@mwri.magee.edu or call 412-641-2400.
Link http://www.AR3T.pitt.edu/atc
SCIENTIFIC ADVANCES
Dr. Peter Rubin Receives Pittsburgh Health Data Alliance Project Funding

In an effort to solve some of healthcare’s toughest challenges through the innovative use of technology, the University of Pittsburgh Medical Center (UPMC) Enterprises announced recently that it will fund several projects created under the umbrella of the Pittsburgh Health Data Alliance. Announced in March 2015, the Pittsburgh Health Data Alliance is a unique collaboration among UPMC, the University of Pittsburgh, and Carnegie Mellon University. It will focus on building new companies that create data-intensive software and services, with the potential to revolutionize health care and wellness.
Through Pitt’s Center for Commercial Applications of Healthcare Data, McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, Chair of the Department of Plastic Surgery, UPMC Endowed Professor of Plastic Surgery, and Professor of Bioengineering at the University of Pittsburgh, will receive funding for his project entitled PUMP. This effort affords a solution aimed at reducing hospital-acquired pressure ulcers, affecting an estimated 3 million patients annually. The monitoring and alert solutions, using wearable devices and hospital bed sensors, will provide real-time documentation of patient repositioning and a process to improve compliance with these preventative measures.
“We are excited to move forward with the first of many exceptional ideas in the Health Data Alliance pipeline,” said Tal Heppenstall, president of UPMC Enterprises. “This promising start bodes well for the Alliance’s goal of transforming health care by unleashing the creativity and entrepreneurialism of leading scientists and clinicians in Pittsburgh.”
ECM May Treat Stroke Patients
McGowan Institute for Regenerative Medicine affiliated faculty member Michel Modo, PhD, associate professor in the Department of Radiology at the University of Pittsburgh with secondary appointments in the Department of Bioengineering and the Center for Neural Basis of Cognition, is the co-author of a paper currently being published by Biomaterials. The title of the paper is “ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate.”
Per Dr. Modo, “As far as we are aware, this is the first time someone demonstrated the infiltration of host brain cells into a biomaterial in the brain.”
McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the Institute, is also a co-author on the study.
The abstract from the paper reads:
Brain tissue loss following stroke is irreversible with current treatment modalities. The use of an acellular extracellular matrix (ECM), formulated to produce a hydrogel in situ within the cavity formed by a stroke, was investigated as a method to replace necrotic debris and promote the infiltration of host brain cells. Based on magnetic resonance imaging measurements of lesion location and volume, different concentrations of ECM (0, 1, 2, 3, 4, 8 mg/mL) were injected at a volume equal to that of the cavity (14 days post-stroke). Retention of ECM within the cavity occurred at concentrations >3 mg/mL. A significant cell infiltration into the ECM material in the lesion cavity occurred with an average of ∼36,000 cells in the 8 mg/mL concentration within 24 h. An infiltration of cells with distances of >1500 μm into the ECM hydrogel was observed, but the majority of cells were at the tissue/hydrogel boundary. Cells were typically of a microglia, macrophage, or neural and oligodendrocyte progenitor phenotype. At the 8 mg/mL concentration, ∼60% of infiltrating cells were brain-derived phenotypes and 30% being infiltrating peripheral macrophages, polarizing toward an M2-like anti-inflammatory phenotype. These results suggest that an 8 mg/mL ECM concentration promotes a significant acute endogenous repair response that could potentially be exploited to treat stroke.
Illustration: Michel Modo, et al.
Redefining Sepsis: An International Effort to Improve Patient Outcomes

UPMC and University of Pittsburgh School of Medicine doctors—including McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, the Dr. Mitchell P. Fink Professor and chair of Pitt’s Department of Critical Care Medicine—played a central role in a worldwide effort to redefine the No. 1 killer of hospital patients: sepsis.
In a series of articles published recently in the Journal of the American Medical Association, an international task force updated definitions of sepsis and septic shock, which were last revised in 2001. These new definitions help bring clarity to sepsis, a syndrome that is difficult for doctors to diagnose and deadly for patients. The task force definitions were directly informed by research out of the Clinical Research, Investigation, and Systems Modeling of Acute illness (CRISMA) Center at the Pitt School of Medicine and were funded in part by grants from the National Institutes of Health.
Sepsis is a condition that arises when the body’s response to an infection injures its own tissues and organs, sometimes progressing to septic shock. It may occur in up to 2 million U.S. patients every year, and, despite best practice, more than 1 in 10 sepsis patients do not survive.
“Considerable advances have been made in the study and care of sepsis and septic shock in the past 15 years, and there is an urgent need to help the medical community do a better job identifying septic patients quickly and start life-saving treatment,” said Dr. Angus. “Put simply, sepsis is a life-threatening organ dysfunction due to a dysregulated response of the patient’s immune system to infection. Our intent is that this definition results in greater consistency for epidemiologic studies, clinical trials, and – perhaps most important – better recognition and more timely management of patients with, or at risk of developing, sepsis.”
“Because sepsis is so complex, patients can present in many different ways. This can lead to delays in care and missed opportunities. The updated definition was crafted to help doctors and hospitals sort through the many sepsis symptoms and get to the diagnosis quickly,” said Dr. Seymour. “Unlike prior attempts to define sepsis, the task force sought data from real patients to inform their deliberations.”
The new criteria include the “quick Sepsis-related Organ Failure Assessment,” or qSOFA, to help clinicians diagnose septic patients and get treatment started outside the intensive care unit. It uses only three symptoms: altered mental status, fast respiratory rate, and low blood pressure, and does not require blood tests. If patients with infection show two of the three criteria, they should be considered likely to be septic. Such patients account for more than 3 out of 4 infection-related deaths. The qSOFA prompt was developed by analyzing more than 800,000 electronic health record encounters at 177 hospitals worldwide, including academic, community, public, private, and federal hospitals.
In addition to Drs. Angus and Seymour, who are lead or senior authors on several of the publications, and Jeremy Kahn, MD, MS, co-author, Pitt Department of Critical Care Medicine, other authors come from Kaiser Permanente, University of Michigan, Veterans Affairs Center for Clinical Management Research, Australian and New Zealand Intensive Care Research Centre, University College London, Guy’s and St. Thomas’ NHS Foundation Trust in the UK, University of Versailles, Jena University Hospital in Germany, Monash University in Australia, Vanderbilt University, Descartes University in France, Emory University, Washington University, Brown University, University of Toronto, Academisch Medisch Centrum in the Netherlands, Erasme University Hospital in Belgium, Steven and Alexandra Cohen Children’s Medical Center in New York, New York State Health Department, Brigham and Women’s Hospital, University of Washington, and Seattle Children’s Research Institute.
Illustration: Dr. Derek Angus (left) and Dr. Christopher Seymour (right), both of the University of Pittsburgh Department of Critical Care Medicine, and others in the Clinical Research, Investigation, and Systems Modeling of Acute Illness Center Laboratory at the University of Pittsburgh School of Medicine discuss sepsis. (Credit: Tim Betler/UPMC.)
A Retinal Prosthesis for the Blind

The Neural Devices Engineering Laboratory, Institute for Complex Engineered Systems at Carnegie Mellon University, is headed by Senior Systems Scientist Shawn Kelly, PhD, a McGowan Institute for Regenerative Medicine affiliated faculty member. At the Neural Devices Engineering Laboratory, Dr. Kelly and his team design and develop novel medical technologies, in particular circuits for neural interfaces. The team primarily designs neural stimulation circuits and inductively-coupled wireless power and data telemetry systems. They also study electrode-tissue interface, as well as the process for inducing nerves to fire, in order to develop optimized, longer-lasting neural interfaces for neural stimulation and recording applications and neuroscience research.
One of the projects in the lab is work on a retinal prosthesis in collaboration with the Boston Retinal Implant Project, a joint effort of the Veterans Administration, Massachusetts Institute of Technology, and Massachusetts Eye and Ear Infirmary, a group Dr. Kelly has worked with since 1996. The retinal prosthesis restores useful sight to people blind with degenerative retinal diseases by stimulating healthy retinal ganglion nerves to create a pixelated image. The group has successfully built three different generations of wirelessly-powered prostheses and implanted all three in animals in preparation for clinical trials. He is the lead electrical system architecture designer for the next generation implant, designing all of the inductively coupled power and data telemetry systems for the prosthesis and coordinating current generation implant component assembly by outside vendors.
As reported by Kathy Serenko, Next Pittsburgh, the retinal prosthesis is an implantable electronic device that bypasses the non-functioning retina by creating and sending electronic signals to the optic nerve. Researchers have spent years tackling a multitude of complex challenges, including the wireless delivery of power to the ocular nerve cells, the implantation of the device in a very small cavity at the back of the eye and the safety of the device over the course of the user’s lifespan.
During initial clinical trials, electrodes were inserted into the eyes of blind people for a few hours to prove the feasibility of an implantable retinal prosthesis. Now, the team is working with the FDA to gain approval for a much more extensive round of clinical trials, a process that could take another 2 years.
In the meantime, the team has reduced the size of the implantable device, created a new and safer titanium case, advanced microchip technology, and fabricated new and improved electrodes.
In the next round of clinical trials, the number of electrodes will be increased from 15 to more than 200, in order to restore a more functional level of vision.
Because natural vision is driven by millions of photo receptor cells, researchers understand that the replacement of a few hundred will result in pixelated images. Restoring enough vision to maneuver safely through the tasks of daily life, however, is reason enough to keep Dr. Kelly committed to his research, even if that means another 20 years.
Illustration: Dr. Shawn Kelly with the retinal prosthesis. –Next Pittsburgh.
Most Patients Likely to See Reductions in Pain and Disability after Bariatric Surgery; Study Identifies Who Benefits Most

In the 3 years following bariatric surgery, the majority of patients experienced an improvement in pain and walking ability, as well as a lessening of the degree to which back or leg pain interfered with work, according a University of Pittsburgh Graduate School of Public Health-led analysis of a multi-site clinical study published recently in the Journal of the American Medical Association. McGowan Institute for Regenerative Medicine affiliated faculty member Steven Belle, PhD, MScHyg, Professor in the Department of Epidemiology, Graduate School of Public Health at the University of Pittsburgh, and a Co-Director in the Epidemiology Data Center in the Graduate School of Public Health, is a co-author on the study.
The study also revealed patient characteristics that indicate who is the most and least likely to experience improvements in pain and function, a finding that could allow clinicians to identify patients who may require additional interventions to improve outcomes. The research was funded by the National Institutes of Health (NIH).
“Our study found that clinically meaningful improvements in bodily pain, specific joint pain, and physical function are common following bariatric surgery. In particular, walking is easier, which impacts patients’ ability to adopt a more physically active lifestyle. However, some patients continue to have significant pain and disability,” said lead author Wendy King, PhD, associate professor in the Department of Epidemiology at Pitt Public Health. “This data can help patients and clinicians develop realistic expectations regarding the impact of bariatric surgery on pain and disability.”
Dr. King and her colleagues followed 2,221 patients participating in the Longitudinal Assessment of Bariatric Surgery-2, a prospective study of patients undergoing weight-loss surgery at 1 of 10 hospitals across the U.S. After 3 years, patients weighed, on average, 28 percent less than prior to surgery. The majority of the patients received Roux-en-Y gastric bypass, a surgical procedure that significantly reduces the size of the stomach and changes connections with the small intestine.
Through 3 years of follow-up, 50 to 70 percent of adults with severe obesity who underwent bariatric surgery reported clinically important improvements in bodily pain, physical function, and usual walking speed. About three-quarters of the participants with symptoms indicative of osteoarthritis before surgery experienced improvements in knee and hip pain and function. In addition, over half of participants who had a mobility deficit prior to surgery did not post-surgery.
“Functional status is an extremely important aspect of health that has not been as well-studied as other conditions that change following bariatric surgery, and this study sheds light on specific factors that may affect improvements in individuals with joint pain who undergo these procedures,” said study co-author Anita Courcoulas, MD, MPH, chief of minimally invasive bariatric and general surgery in Pitt’s School of Medicine.
Study of Enzymatic Chemical Reactions by Pitt and Penn State Researchers May Indicate How the First Cells Formed Colonies
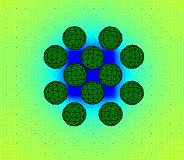
A novel investigation of how enzymatic reactions can direct the motion and organization of microcapsules may point toward a new theory of how protocells – the earliest biological cells – could have organized into colonies and thus, could have ultimately formed larger, differentiated structures.
Researchers at the University of Pittsburgh’s Swanson School of Engineering, along with collaborators at Penn State University’s Chemistry Department, found that very simple physical and chemical processes that do not rely on complex biological machinery guided the self-assembly of the microcapsules, which served as models for the protocells. Namely, the researchers isolated a dynamic cascade of events that lead the microcapsules to organize into a well-defined colony.
McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering at Pitt, with post-doctoral associates Oleg E. Shklyaev, PhD, and Henry Shum, PhD, developed the computational modeling based upon previous experiments conducted by Ayusman Sen, PhD, Distinguished Professor of Chemistry at Penn State University. Their paper, “Harnessing surface-bound enzymatic reactions to organize microcapsules in solution,” was published recently in the AAAS journal Science Advances.
The researchers modeled microcapsules between 10-50 micrometers in diameter, the typical size of biological cells. In this study, the microcapsules consisted of an outer shell and a fluid-filled core containing hydrogen peroxide, which gradually leaked through the shell into the surrounding fluid. The hydrogen peroxide acted as a chemical reagent for a patch of enzymes on the surface under the microcapsules. The reaction occurring at the enzymes released heat and lowered the fluid density, driving the convection of the surrounding fluid. This fluid flow carried the immersed capsules and brought them together above the enzyme-coated surface. After the reagent was consumed, the fluid flow ceased and the capsules remained localized above the patch of enzymes.
According to Dr. Balazs, this research provides a novel approach for manipulation in small fluidic devices. Utilization of different catalysts would allow different flow patterns to develop depending on the chemicals present in the fluid or microcapsules. This could potentially lead to autonomous sorting of cells or assembly of large, predesigned structures from smaller building blocks.
Illustration: Chemically-generated convection provides means of organizing protocells into colonies and microcapsules on surfaces. University of Pittsburgh.
Nutrition and Surgical Site Infections: Study

As reported by Susan M. Rapp for Healio, spine surgery patients at the University of Pittsburgh Medical Center (UPMC) who had impaired preoperative nutrition based on levels of prealbumin less than 20 mg/dL had an increased risk of developing a surgical site infection, according to McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD, at Spine Summit 2016: CNS/AANS Section on Disorders of the Spine & Peripheral Nerves Annual Meeting.
Dr. Okonkwo, Professor and Executive Vice Chair of Neurological Surgery, University of Pittsburgh, Professor of Sports Medicine and Nutrition, Director of Neurotrauma and of the Scoliosis and Spinal Deformity Program at UPMC, and Clinical Director of the Brain Trauma Research Center, and colleagues prospectively followed 622 patients in a registry who underwent spine surgery between October 2009 and December 2014 at UPMC. They performed a linear regression analysis to look at the relationship of the patients’ prealbumin level to whether they developed a surgical site infection (SSI).
An earlier retrospective study the investigators conducted showed prealbumin less than 20 mg/dL and a diagnosis of diabetes mellitus were associated with an increased risk of SSI, Dr. Okonkwo said.
“In this prospective study, diabetes mellitus dropped out and a prealbumin of less than 20 remained the sole predictive factor with a hazard ratio of 4.95 of predicting risk for the development of a SSI with a very strong P value,” he said.
The patients, who were tracked with standardized outcome measures, underwent various types of spine surgery. For the analysis, however, cases of deformity correction were excluded, as were lateral interbody fusions, discectomies, and anterior cervical discectomy and fusion.
As for the exclusions, “deformity has an outlier of SSI rates and these others are clearly at a lower risk,” Dr. Okonkwo said.
“We went from retrospective prealbumins at the time of the infection to retrospective of prealbumins taken in the month prior to surgery, to a prospective evaluation of what is the relationship between preoperative nutritional status and SSI. This relationship is born out every single time, which is why we are now developing an intervention program for at-risk patients with poor nutrition profiles to encourage and facilitate a further reduction of the infection rate at our institution,” Dr. Okonkwo said.
AWARDS AND RECOGNITION
Dr. Paul Monga to Lead New Multidisciplinary Liver Center

University of Pittsburgh School of Medicine reports the creation of a new Multidisciplinary Liver Center under the leadership of Satdarshan (Paul) Singh Monga, MD, a McGowan Institute for Regenerative Medicine faculty member. In combination with UPMC’s longstanding commitment to state-of-the-art clinical care for patients with an array of liver diseases, the School of Medicine is leading novel translational and basic research in many aspects of hepatic pathobiology. Building on these existing strengths, the mission of the Liver Center will be to promote transdisciplinary research in Hepatology with a focus on liver development, growth, metabolism, injury, and repair with the expressed goal of improving the health of patients with liver disease. The Liver Center will provide a platform to enhance, enrich, and synergize scientific interactions and collaborations between various investigators in the field of liver pathobiology that are currently residing in diverse departments. The Liver Center will provide mentoring and career development opportunities, especially to early career investigators studying liver health and disease.
Dr. Monga is Vice Chair and Division Chief of Experimental Pathology in the Department of Pathology and Professor of Medicine in the Division of Gastroenterology, Hepatology and Nutrition. He serves as Assistant Dean and Co-Director of the Medical Scientist Training Program at the School of Medicine. He leads the Division of Experimental Pathology, which is currently composed of 20 full-time faculty. He is a member of the American Association of Clinical Investigators and has received many awards in mentoring as well as in research, including the Outstanding Investigator Award from the American Society of Investigative Pathology (ASIP). He has served in key leadership roles at the ASIP, the American Association of Study of Liver Diseases and other societies. He is the current Editor-in-Chief of Current Pathobiology Reports and of Gene Expression: The Journal of Basic Liver Research and Associate Editor for the Journal of Hepatology, American Journal of Pathology, JCI Insights, Seminars in Liver Diseases and Annual Reviews of Pathology.
Dr. Monga is an internationally recognized leader in the field of liver pathobiology with more than 90 original research publications. His work has characterized the role of Wnt/b-catenin signaling in the processes of liver regeneration and hepatic tumors, like hepatocellular cancer and hepatoblastoma, and in hepatic development. This work forms the basis for a modulating role of b-catenin signaling in liver regeneration, end stage liver disease, and in the setting of liver transplantation. Dr. Monga has been continuously funded by the NIH since 2003 and is currently PI on four R01 awards and two corporate research agreements with Abbvie and Dicerna. He is also the director of an NIH-funded predoctoral training program (T32), entitled Cellular Approaches to Tissue Engineering and Regeneration (CATER), at the McGowan Institute.
Paper Wins International Dupuytren Award 2016

The Scientific Advisory Council of the International Dupuytren Society selected a paper by McGowan Institute for Regenerative Medicine affiliated faculty member Latha Satish, MSc, MPhil, PhD, as the winner of its International Dupuytren Award 2016 in the category Basic Research. The paper is entitled “Developing an animal model of Dupuytren’s disease by orthotopic transplantation of human fibroblasts into athymic rat” and appeared in the journal, BMC Musculoskeletal Disorders. McGowan Institute for Regenerative Medicine affiliated faculty member Sandeep Kathju, MD, PhD, is a co-author on the study.
From the abstract, Dupuytren’s disease (DD) is a slow, progressive fibroproliferative disorder affecting the palms of the hands. The disease is characterized by the formation of collagen rich- cords which gradually shorten by the action of myofibroblasts resulting in finger contractures. It is a disease that is confined to humans, and a major limiting factor in investigating this disorder has been the lack of a faithful animal model that can recapitulate its distinct biology. The aim of this study was to develop such a model by determining if Dupuytren’s disease (DD)- and control carpal tunnel (CT)-derived fibroblasts could survive in the forepaw of the nude rats and continue to exhibit the distinct characteristics they display in in vitro cultures.
The International Dupuytren Award recognizes exceptional scientific publications on research or clinical treatment of Dupuytren and/or Ledderhose disease. Research can be about treatment, epidemiology, pathogenesis, genomics, or other areas, like anatomy, improving the understanding and treatment of these diseases.
Dr. Satish is a Research Assistant Professor in the Department of Plastic Surgery at the University of Pittsburgh. Dr. Kathju is an Associate Professor with the University of Pittsburgh School of Medicine in its Department of Plastic Surgery. He also serves as the Chief of Plastic Surgery and the Medical Director of the Wound Healing Center, both appointments at UPMC Passavant Hospital. In addition, he is the Co-Medical Director of the Wound Healing Center at UPMC McKeesport Hospital.
Illustration: Dupuytren’s contracture of the ring finger. Wikipedia.
Dr. Steffi Oesterreich Joins Susan G. Komen As Advisor, New Komen Scholar

Susan G. Komen, the world’s leading breast cancer organization, welcomes McGowan Institute for Regenerative Medicine affiliated faculty member Steffi Oesterreich, PhD, Professor of Pharmacology and Chemical Biology at the University of Pittsburgh Cancer Institute, and 15 other leaders in breast cancer research and advocacy who will serve as Komen Scholars – an international advisory group which helps to guide Komen’s research and scientific programs, education and advocacy work, and public health efforts in the U.S. and abroad.
Says Dr. Oesterreich, “I’m very happy and honored to be part of this group of 16 people from all over the world. I want to support the Komen mission, which is to reduce mortality from breast cancer.”
The new members join 44 current Komen Scholars who since 2010 have advised and provided expertise to Komen in a variety of capacities, including a primary responsibility of leading and participating in the annual peer-review process that Komen uses to fund its research program. At $889 million since 1982, Komen’s is the largest breast cancer research investment of any nonprofit.
Komen Scholars are chosen for their knowledge and leadership within the scientific, research and advocacy communities, and for their own contributions to breast cancer research. Scholars bring a wide range of proficiencies across the spectrum of breast cancer science and care, including metastatic breast cancer, immunology, prevention, public health, disparities, and patient advocacy.
“The Komen Scholars are a brilliant group of cancer researchers who bring a wealth of knowledge and experience to our collective fight against this disease,” said Komen Chief Scientific Advisor Dr. George Sledge. “Their expertise goes from the bench to the bedside, and many have already made important contributions to our understanding of the disease, and to its conquest through better diagnosis and treatment.”
Dr. Shilpa Sant Receives BMES Rising Star Award

McGowan Institute for Regenerative Medicine affiliated faculty member Shilpa Sant, PhD, received a Rising Star Award for Early Career Faculty from the Biomedical Engineering Society during the 2016 Biomedical Engineering Society-Cellular and Molecular Bioengineering meeting held in New Orleans, Louisiana.
This year’s conference theme focused on engineered tissues as models of disease, micro- and macro-scale technologies for building tissues, mechanobiology of regenerative medicine, and stem cell processing.
Dr. Sant delivered her presentation entitled “Engineering Tumor Microenvironments in Early and Advanced Stage Breast Tumors.” Previously, Dr. Sant was the recipient of a Rising Star Award in the postdoctoral fellow category on her work on gradient materials for tooth morphogenesis in 2013.
Dr. Sant is an assistant professor in the Department of Pharmacy at the University of Pittsburgh.
Congratulations, Dr. Sant!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#158 –– Elisabeth Barton, PhD is a Professor in the Department of Applied Physiology and Kinesiology at the University of Florida. Dr. Barton discusses her research in skeletal muscle repair.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
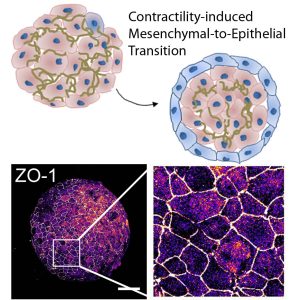
Mesenchymal-to-epithelial transition in 3D embryonic aggregates. Surface cells on pluripotent mesenchymal aggregate adopt apical-basal polarity and transition to epithelia cells (ZO-1) in response to cell contractility. Pseudo-colored ZO-1 expression shows scattered clusters of epithelial cells across the surface of the mesenchymal-cell aggregate (H. Y. Kim, T. R. Jackson, and L. A. Davidson). Scalebar 250 µm.
PUBLICATION OF THE MONTH
Author: Ghuman H, Massensini AR, Donnelly J, Kim SM, Medberry CJ, Badylak SF, Modo M
Title: ECM hydrogel for the treatment of stroke: Characterization of the host cell infiltrate
Summary: Brain tissue loss following stroke is irreversible with current treatment modalities. The use of an acellular extracellular matrix (ECM), formulated to produce a hydrogel in situ within the cavity formed by a stroke, was investigated as a method to replace necrotic debris and promote the infiltration of host brain cells. Based on magnetic resonance imaging measurements of lesion location and volume, different concentrations of ECM (0, 1, 2, 3, 4, 8 mg/mL) were injected at a volume equal to that of the cavity (14 days post-stroke). Retention of ECM within the cavity occurred at concentrations >3 mg/mL. A significant cell infiltration into the ECM material in the lesion cavity occurred with an average of ∼36,000 cells in the 8 mg/mL concentration within 24 h. An infiltration of cells with distances of >1500 μm into the ECM hydrogel was observed, but the majority of cells were at the tissue/hydrogel boundary. Cells were typically of a microglia, macrophage, or neural and oligodendrocyte progenitor phenotype. At the 8 mg/mL concentration, ∼60% of infiltrating cells were brain-derived phenotypes and 30% being infiltrating peripheral macrophages, polarizing toward an M2-like anti-inflammatory phenotype. These results suggest that an 8 mg/mL ECM concentration promotes a significant acute endogenous repair response that could potentially be exploited to treat stroke.
Source: Biomaterials. 2016 Jun;91:166-81. doi: 10.1016/j.biomaterials.2016.03.014. Epub 2016 Mar 18.
GRANT OF THE MONTH
PI: J. Peter Rubin, MD
Title: PUMP
Description: PUMP is a solution aimed at reducing hospital-acquired pressure ulcers, affecting an estimated 3 million patients annually. The monitoring and alert solutions, using wearable devices and hospital bed sensors, will provide real-time documentation of patient repositioning and a process to improve compliance with these preventative measures.
Source: Pittsburgh Health Data Alliance
